How to Interpret Your Soil Analysis
27 March 2024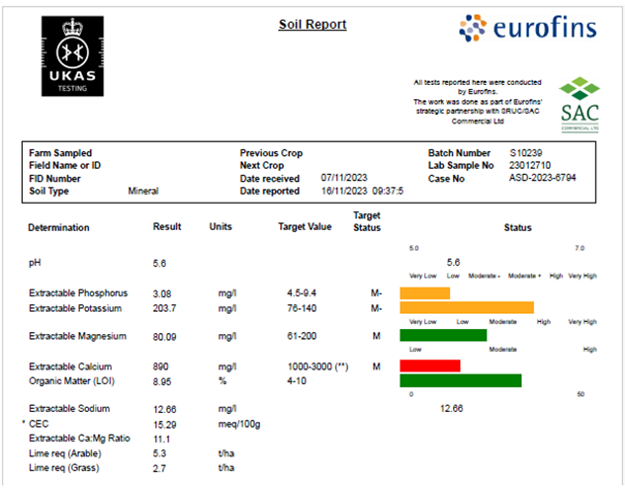
Understanding the information you receive when you have your soils analysed can help to build up the picture of the unique physical and chemical properties of your soils that will affect crop growing conditions on your farm. This article will break down the information that you receive when you run a routine soil analysis and what this means regarding your soils and your agronomic decisions.
Understanding pH
pH is a measure of the number of Hydrogen (H+) ions[1][1] in the soil. When the concentration of H+ ions is high the conditions are acidic and at low concentrations of H+ ions the conditions are alkaline. The target pH for arable/ rotational grass is 6.2 and the target for permanent pasture is grassland is 5.8 or higher. The lime recommendations are the amount of lime required to bring the soil up to the target pH. If growing pastures with a high legume content the target will be higher as the optimum pH for most legumes is 6.0-6.5. The specific pH target can also vary with crop type and soil type. Some of the soil properties affected by pH include the availability of nutrients, the rate of decomposition of organic material and the soil organism species present. Consequently, different crop species have varying tolerances to different soil pH conditions.
[1] 1. An ion is an atom or molecule with an electric charge due to the loss or gain of an electron. Positively charge ions have a positive charge due to the loss of an electron and negatively charge ions have gained an electron.
Extractable Phosphorus
This is the proportion of phosphorus in the soil that can be removed by using different types of chemical extractants. Laboratories have developed extractants to remove specific forms of Phosphorus from the soil to provide an indication of how much phosphate is likely to be available to a growing crop.
PSC Class
PSC is not listed on the soil results but is important to know in order to use the results effectively. PSC Class is the differing capacity of soils to bind with applied phosphate. Phosphate in soils can react with compounds in the soil and get ‘locked up’ in complex molecules. This makes it unavailable for plant uptake. This means that less phosphate application is required for a PSC 1 soil compared to a PSC 3 soil. You can find the PSC class of your soil using the Scotland’s soils interactive map. Home | Scotland's soils (environment.gov.scot). Phosphate availability is also affected by pH. The optimum pH for phosphate availability is between 6.0 and 7.0. At lower or higher pHs phosphate is more likely to react with other compounds in the soil and become unavailable for plant uptake.
Extractable Potassium
This is the proportion of the potassium present that is available for plant uptake. Again, like phosphorus this is tested by using chemical extractants to determine how much might be available for plant uptake within the soil.
Phosphorus and Potassium 'Status'
For cereal based arable rotations the target soil status is moderate. The target for potatoes is moderate plus. On moderate status soils the recommendation is to apply ‘maintenance’ amounts, or in other words, enough to compensate for offtake. In low status soils the target will need to be more than maintenance to adequately supply the growing crop.
Extractable Magnesium and Calcium
Calcium and magnesium are needed in lesser quantities than the macro nutrients N, P and K. Magnesium is the central ion within the chlorophyll molecule which is essential for photosynthesis. Potassium can be antagonistic to the uptake of calcium and magnesium. Therefore, it is important that potassium is not over-supplied as luxury uptake can inhibit the plant from taking up sufficient amounts of other essential cation (positively charged ions) nutrients. This is especially important when growing silage or spring grass as low magnesium levels within the plant can lead to staggers.
LOI
LOI stands for loss on ignition. Loss on ignition is the change in soil mass after combustion which is taken as a representation of the organic matter component of the soil. It is generally assumed that organic matter is 58% carbon and so the organic carbon can be crudely estimated from LOI. More specific tests of soil carbon are also available such as the dumas test.
CEC
Cation Exchange Capacity is a measure of the negative charges within a soil upon which plant nutrient cations can be retained via adsorption. Adsorption is when molecules adhere to a solid surface from a gas, liquid or solution. The attraction between the positive charges on the nutrient ions to the negative charges on the soil surface enables this adhesion and consequently the retention of the nutrient ions within the soil. CEC surface is comprised of clay particles and organic matter particles (particularly humic[2] substances) which have negative charges on their surface. Important plant nutrient cations include K+ potassium, NH4+ ammonium, Ca2+ calcium, and Mg2+ magnesium. These ions are exchanged from the CEC surface with H+ (hydrogen) ions and other cations in response to conditions within the soil solution to be made available for plant uptake. CEC is reported in meq/100g which stands for milliequivalents per 100 grams of soil. Sandy soils will have a lower CEC than clay soils and soils higher in organic matter/ humic content will have a higher CEC also. Therefore, soils with a low CEC and a low potassium status could benefit from organic matter inputs to increase the CEC and therefore increase the efficacy of potash applications.
Extractable sodium is included but this is not a major plant nutrient. It has some effect on the soil pH properties but is otherwise not very relevant to crop growth.
Pointers for where to find more info can be found in one of our technical notes:
Technical Note (TN714) Liming Materials and Recommendations
[2] 2. Humus is organic material that has undergone partial decomposition by soil organisms. It is highly stable and can persist within the soil for a long period of time.
Freya Lance, SAC Consulting
Sign up to the FAS newsletter
Receive updates on news, events and publications from Scotland’s Farm Advisory Service


